Microbes on the Mind: A Complex Role in Neurodegeneration
One evolving plot twist in the story of research into the aging brain is the discovery that neurological health involves much more than the brain, or even other human cells. Enter: microbes.
Humans are living longer, and with that, suffering a higher burden of neurodegenerative diseases. These conditions span a variety of presentations, from progressive cognitive decline to loss of motor control, but share 2 defining features: advanced age and neuroinflammation.
The role of bacteria, viruses and even fungi in neurodegeneration remains shrouded in uncertainty. Are they friend or foe? The answer unfolding from the scientific literature: it depends.
On the one hand, microbes are at the fulcrum of our metabolic wellbeing and immunological development. A healthy coterie of microbes can mediate our protection from brain-damaging systemic inflammation and mediate beneficial effects from our diet and lifestyle. On the other hand, some opportunistic microbes can establish themselves in tissues where they don’t belong — like the brain — and stir the immune system into a damaging response. With age as the primary risk factor for neurodegeneration, it may be that one’s risk is a function of how many immunological insults you have sustained throughout your lifetime.
The Biology of Neurodegenerative Disease
Neurodegeneration follows multiple pathologies, of which Alzheimer’s Disease is by far the most prevalent, followed by Parkinson’s Disease. Alzheimer’s Disease is characterized by progressive cognitive decline and memory loss, accompanied by extracellular amyloid beta (Aβ) plaques in the brain and intracellular neurofibrillary tangles made of the microtubular protein tau. Parkinson’s Disease presents with progressive loss of motor control, leading to physical tremors, rigidity and difficulty with muscle control. These symptoms develop from the death of dopamine-producing neurons in the substantia nigra of the brain and the development of protein aggregates made of α-synuclein called Lewy bodies.
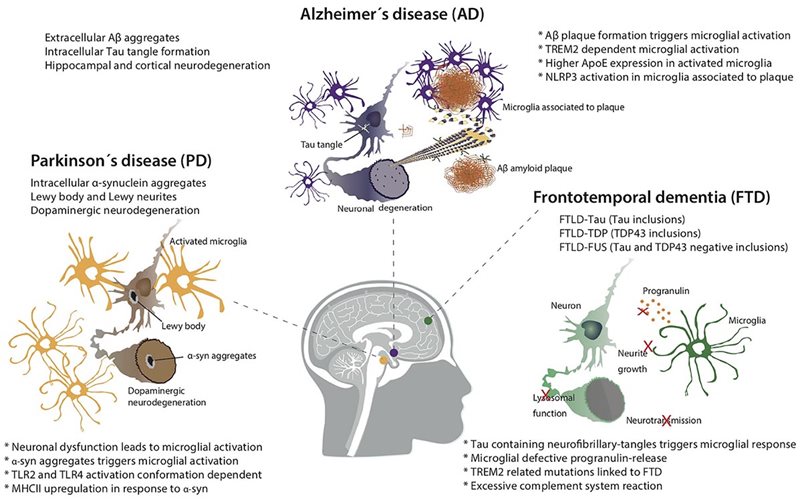
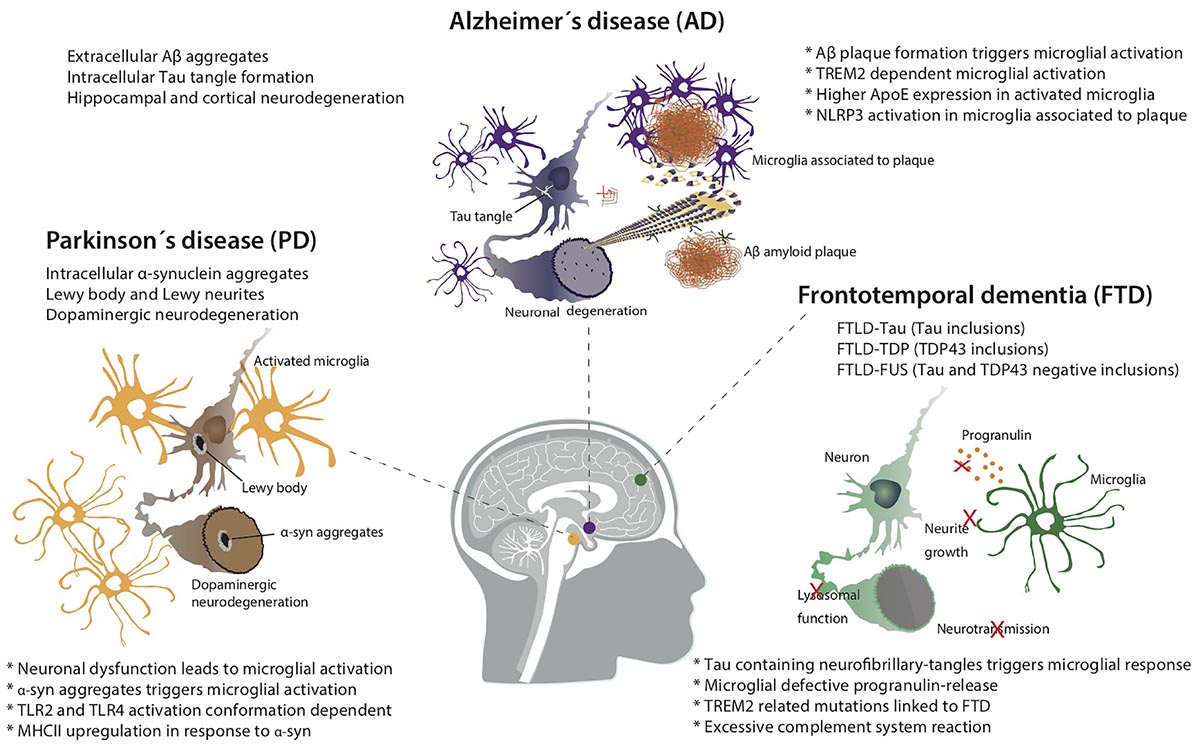
In both pathologies—as in other neurodegenerative conditions—neuroinflammation is at the core of disease progression. Microglia are the resident immune cells of the brain, carrying out surveillance and clearance functions. Their hyperactivation is implicated in multiple neurodegenerative conditions, including Alzheimer’s Disease, Parkinson’s Disease, amyotrophic lateral sclerosis (ALS) and other forms of dementia. When triggered by nearby danger signals, microglia activate and release reactive oxygen species (ROS), nitric oxide (NO) and inflammatory cytokines into the region. If prolonged, this reaction can damage nearby neurons, which in turn secrete danger signals of their own. It is a treacherous positive feedback loop.
The Infection Hypothesis of Neurodegenerative Disease
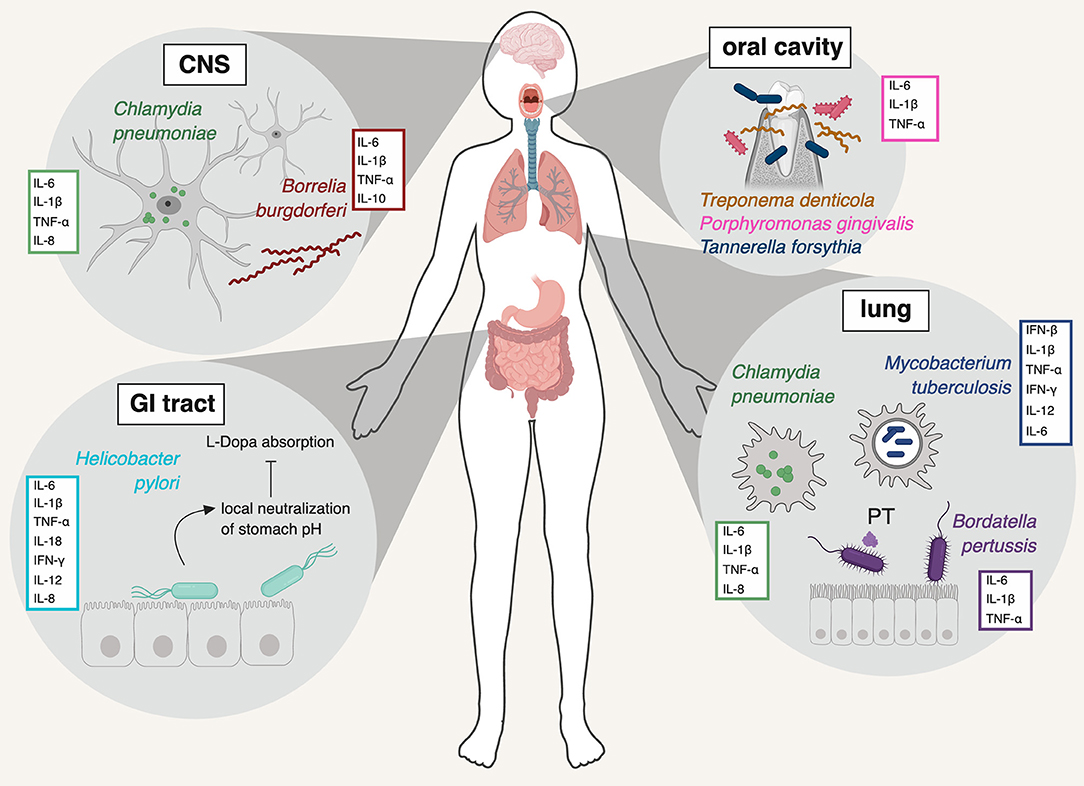
There is some evidence to suggest that specific microbes can gain access to the brain and trigger an inflammation cascade that may ultimately be involved in neurodegeneration. This hypothesis emanates from data that localizes viruses, bacteria and even protozoa in the brains of people with neurodegenerative disease, and recognizes similar biomarker patterns of infection to forms of dementia. Nested within the hypothesis of infection as a driver of neurodegeneration, the “Antimicrobial Protection Hypothesis” reframes deposition of protein aggregates, such as Aβ, as innate immune responses to microbial invasion, rather than an intrinsically pathological aspect of the disease. Although no specific pathogen has been definitively incriminated in the development of neurodegenerative disease, the hypothesis opens up the possibility that infection, in general, may be the inflammatory insult that progresses neurodegenerative pathology.Viruses
Viruses like herpes simplex virus 1 (HSV-1) have co-evolved with humans for a long time. They are prevalent in the population and establish long-term latency within their hosts, including in the brain. However, HSV-1 has been found in the post-mortem brains of people with Alzheimer’s Disease at a significantly higher rate than people without Alzheimer’s. HSV-1 DNA can even be found in the Aβ plaques of those with Alzheimer’s. In mice, repeated infection with HSV-1 can create a progressive Alzheimer’s-like pathology. Notably, HSV-1 evades the neural immune system by inhibiting autophagy, which is a critical function for the clearance of damaged organelles, protein aggregates and cellular debris. Autophagy is dysfunctional in many neurodegenerative conditions. However, HSV-1 is also a highly-prevalent virus, suggesting that other factors are at play.The Coronavirus Disease 2019 (COVID-19) pandemic has also sparked discussion around the neurological impacts of viral infection. Although it is too soon to know whether sudden acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the viral agent of COVID-19, can precipitate neurodegenerative disease, neurological effects have been observed in a subset of people who acquire the disease. The receptor it uses to invade cells, angiotensin converting enzyme-2 (ACE2), is expressed in relatively high amounts in the central nervous system, although there are multiple possible routes of viral access to the brain, such as within infected leukocytes or through a compromised blood-brain barrier (BBB). SARS-CoV-2 has been found in the neurons of some post-mortem brains of people with COVID-19, although neurons do not appear to be its preferential site of infection. Research does show that people with existing dementia are more likely to have severe outcomes of COVID-19, and some researchers have already begun to speculate that COVID-19 infection may put some individuals at greater risk for subsequent neurodegenerative disease. It’s worth noting, though, that these associations may precipitate from shared risk factors for both COVID-19 and dementia, such as obesity or type II diabetes.
Bacteria and Protozoa
Periodontitis is a chronic inflammatory condition of the gums and mouth. Oral bacteria, such as Porphyromonas gingivalis, have been associated with periodontitis and an increased risk for Alzheimer’s Disease, making the bacteria of the oral cavity a site of interest in understanding disease pathology. However, more clarifying data is needed to establish this connection.The respiratory pathogen Chlamydia pneumoniae is significantly affiliated with neurodegenerative disease. In one study of post-mortem brains, it was found in 90% of people who had late-onset Alzheimer’s, compared to only 5% of healthy brains, and this association was repeated in a later study by another group. Cell staining in the brains of patients frequently identified C. pneumoniae in cells that were close to neurofibrillary tangles and Aꞵ plaques.
Toxoplasma gondii is a common parasite that can establish itself in the human brain as a life-long host. It, too, is affiliated with neurodegeneration, both in animal models of wild-type mice and in the global human population.
Bacterial Amyloids
Although one view of amyloids is that they are an innate immune mechanism, amyloid-like fibers are also produced by microbes themselves in the development of biofilms. It is possible that plaques in the brain are themselves biofilms created by microbes attempting to shield themselves from immunological destruction. Many normal members of the human microbiota are capable of forming bacterial amyloids, such as Streptococcus, Staphylococcus, Klebsiella, Citrobacter and Escherichia coli. In animal models, exposure to amyloid-producing E. coli increased α-synuclein production in the gut and accumulation in the brain. Even animals that over-express α-synuclein are not able to form Lewy bodies and the associated pathology without the presence of microbes, suggesting an important role for bacteria in Parkinson’s Disease. Studies suggest that bacterial amyloids may be able to cross-seed in prion-like fashion, promoting enhanced production of amyloid from diverse species of bacteria. Such fibers can also drive an immune response through activation of inducible nitric oxide synthase (iNOS) and nuclear-factor kappa-B (NF-κB).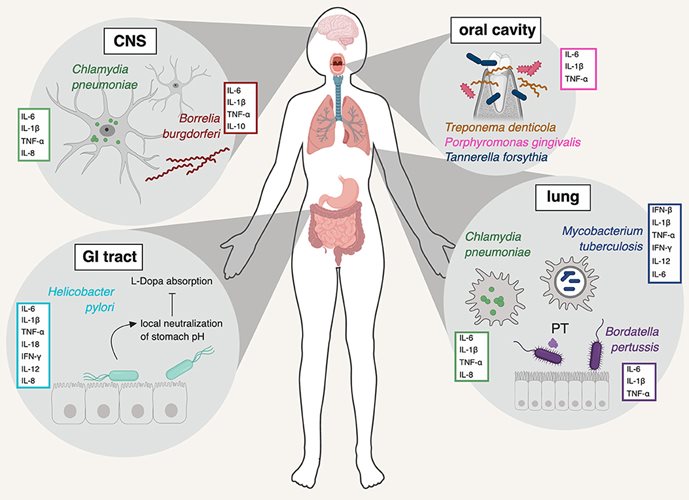
The Microbiota, Lifestyle and Neuroprotection
The infection hypothesis has come of age alongside our broadening understanding of the human microbiota, or the vast assemblage of mutualistic microorganisms that live on and inside of us. This symbiotic body of microbes is critically important for our immunological development, and takes part in the daily activity of transforming the foods we eat into useful metabolites. Some of the most important metabolites produced by bacteria in our gut are short-chain fatty acids (SCFA), which are produced when bacteria break down indigestible fibers from our diet. These compounds, especially butyrate, are potent anti-inflammatory signaling molecules that reinforce a strong colonic barrier, reprogram the immune system into an anti-inflammatory state and even calm down microglia. Butyrate helps reinforce the BBB (which is frequently disrupted during neurodegeneration), and direct administration of butyrate in water was able to delay the onset of amyotrophic lateral sclerosis (ALS) in mice. All of these effects become accessible to us through the dietary intake of fiber, and microbial assistance in breaking it down.If the microbiota becomes imbalanced, a state referred to as ‘dysbiosis,’ systemic inflammation can ensue. Inflammation may also exert its own effect on the microbiota as well. In states of overnutrition from high fat, high sugar, low fiber diets (such as the Western diet), fiber-digesting bacteria lose out to other, less-beneficial bacteria and butyrate production drops. In mice, high-fat, diet-induced changes in the microbiota were significantly associated with deficits in memory, even without changes to inflammatory cytokine expression. Moreover, different neurodegenerative diseases share some common gut microbial signatures with each other, often affiliated with a reduced abundance of fiber-fermenting (butyrate-producing) bacteria.
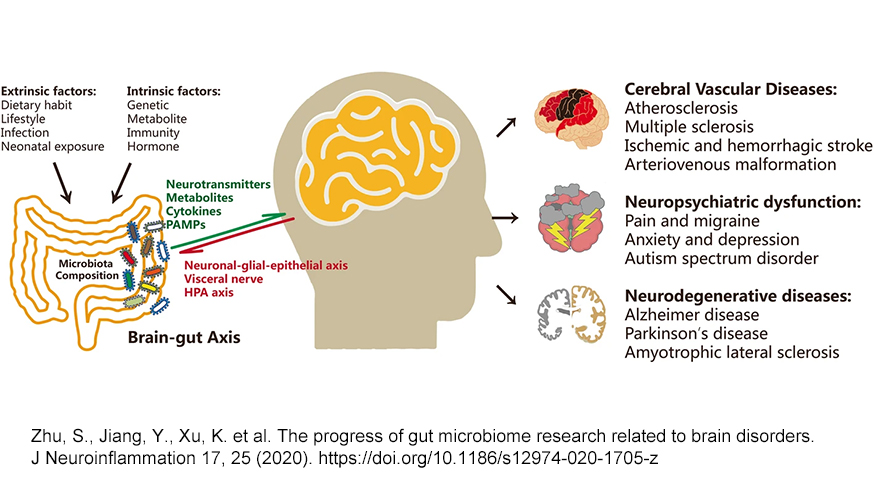
Putting It All Together
If neurodegenerative diseases were primarily caused by infection, we would expect to see their prevalence be the highest in places that have a higher burden of infectious disease. Some of the key viruses discussed, like HSV-1, have the highest prevalence in regions like Africa and Southeast Asia. However, these regions have a markedly lower incidence of dementia than regions with a higher burden of vascular metabolic disease, such as Latin America. Although factors like life expectancy at birth or even the availability of data may also play a role, these data could imply that context may be an important factor in the neuropathogenesis of microbes.Metabolic diseases and the unhealthy microbiota that accompany them still occur at higher rates in North America, Europe and increasingly Latin America (although epidemiology is shifting). An imbalanced microbiota creates a vulnerability to inflammation, a leaky gut and a permeable BBB. It’s feasible that infection from opportunistic pathogens in the context of a weakened blood brain barrier, or heightened systemic inflammation, may allow critical access to the brain for otherwise innocuous symbionts. By another token, currently declining rates of dementia in high-income countries that are not fully explained by changes in metabolic health could suggest that lower rates of infection in these regions have indeed been neuroprotective. There are also genetic factors in a subset of those who go on to develop neurodegenerative disease that we have not discussed here, but are affiliated with a greater vulnerability to central nervous system infection.
What appears to surface from the data is that neuroinflammation is the lynchpin. How you get to that inflammatory state may be secondary. It may be that repeated exposures to pathogens that cause neuroinflammation cross a critical threshold. Indeed, a study on Parkinson’s showed that exposure to a greater variety of pathogens was affiliated with increased disease risk. It may also be that uncontrolled systemic inflammation via one’s diet and lifestyle, as mediated by the microbiota, drives a neuroinflammatory state. Data support that framework as well. More likely, a combination of these and other factors is at play. Perhaps in the case of neurodegenerative disease and microbes, many paths may converge on the same pathology.
Although we may not always be able to control the infectious agents we are exposed to (something we feel acutely in the COVID-19 era), we can do our best to harness the lifestyle-responsive microbes that reside in our gut. Research repeatedly shows that lifestyle factors like diet, exercise, stress and sleep can have profound effects on neurological outcomes, and these effects are mediated, in part, by the good microbes in our lives. Perhaps by strengthening our gut microbe communities we can not only tamp down on systemic inflammation and the diseases it promotes, but also help protect our brains from harmful microbes that might otherwise get an opportunistic foothold in our brains.
Further Reading:
- Exploring the “Multiple-Hit Hypothesis” of Neurodegenerative Disease: Bacterial Infection Comes Up to Bat
- The Antimicrobial Protection Hypothesis of Alzheimer’s Disease
- Can an Infection Hypothesis Explain the Beta Amyloid Hypothesis of Alzheimer’s Disease?
- Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases
- The Microbiome as a Modifier of Neurodegenerative Disease Risk
- Microglia-mediated neurotoxicity: uncovering the molecular mechanisms
- Butyrate, neuroepigenetics and the gut microbiome: Can a high-fiber diet improve brain health?
- Gut microbial molecules in behavioural and neurodegenerative conditions
- Can an Infection Hypothesis Explain the Beta Amyloid Hypothesis of Alzheimer’s Disease?
