The Gut Microbiota in Action: Tackling Celiac Disease

Source: kilonad/flickr.com
What is Celiac Disease?
The National Institute of Health (NIH) defines CeD as a persistent gastro-immunological disorder that impacts the integrity of the small intestine. The condition occurs when the immune system of people with certain genetic predispositions erroneously considers gluten (and similar dietary proteins) a threat and stimulates an immune response against them. Essentially, when gluten is consumed by a in individual who has CeD, the small intestine becomes a battleground. The result? Damages to the villi of the small intestine—finger-like projections responsible for nutrient absorption—leads to impaired nutrient absorption and can trigger a diverse range of symptoms that vary in severity and encompass gastrointestinal, dermatological, neurological, musculoskeletal, reproductive, hormonal, psychological and behavioral manifestations.CeD generally occurs in genetically predisposed individuals. Notably, over 30% of the global population carries the genetic markers associated with CeD. However, only 2-3% of individuals develop the disease in their lifetime. This suggests that other physiological and/or environmental factors, such as gut microbiome health, are involved in the etiology and pathogenesis of CeD.
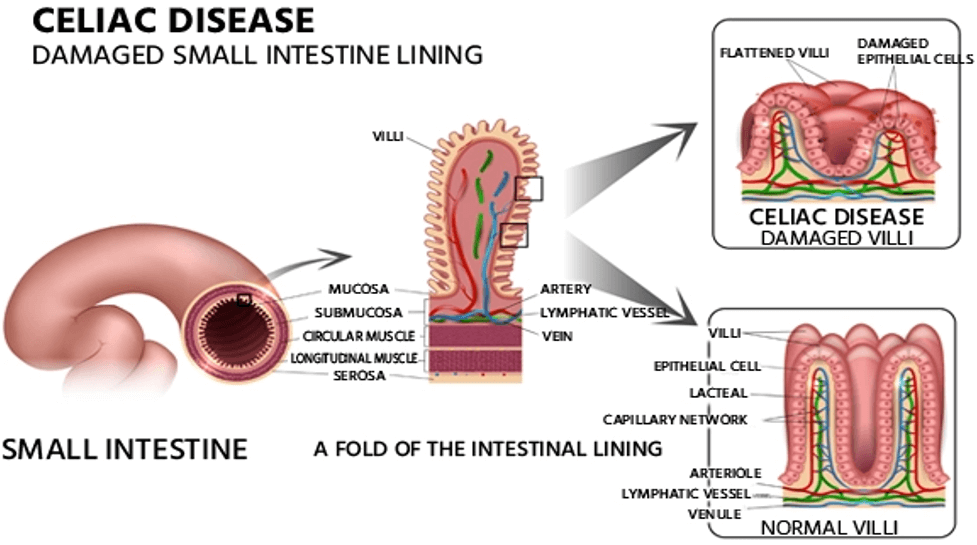

Source: TefIM/iStock.com
Link Between the Gut Microbiota and Celiac Disease
A healthy gut microbiota—the community of microbes living in the gastrointestinal tract—is crucial for maintaining host health, particularly in protecting against various pathogens. The microbiota also regulates the normal development, structure and physiology of the gastrointestinal tract.Furthermore, studies have shown that gut commensals are involved in the metabolism of gluten, suggesting that the gut microbiota employs different mechanisms to protect against CeD. Gliadin, a highly immunogenic and major component of gluten that is implicated in CeD, is broken down by enzymes of gut commensals, thus reducing inflammation, preventing intestinal damage and improving intestinal permeability.
To that end, the innate immune system has a major role in initiating the immune response underlying CeD, inducing mucosal injury and boosting the adaptive immunological response. The receptors responsible for the interaction between the mucosal wall of the gut and intestinal microbiota are the same as those required for activation of innate immunity and include Toll-like receptors (TLRs) and Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs). As a result, any change in microbiome composition from a beneficial to pathogenic state can alter the availability of TLR ligands, leading to dysregulated TLR activation, and aberrant TLR signaling in CeD can disrupt the balance between tolerance and inflammation. Changes in NLR activity, due to dysbiosis in the gut, can also disrupt this balance, contributing to the excessive immune response to gluten that occurs in CeD.
A number of studies have undertaken the task of further solidifying the link between microbial dysbiosis and active CeD. A 2021 study examined the trajectory of the gut microbiota of 10 infants who developed CeD (starting 18 months prior to disease onset) and 10 infants who did not develop the autoimmune disease. Here researchers observed that the abundance of bacterial strains that have been previously linked to autoimmune and inflammatory conditions, such as Dialister invisus, Parabacteroides sp. and Lachnospiraceae, increased before disease onset, while the abundance of other species that have been linked to anti-inflammatory effects (e.g., Streptococcus thermophilus, Faecalibacterium prausnitzii and Clostridium clostridioforme) decreased before disease onset.
Another study analyzed the fecal microbiota of healthy, full-term newborns with at least 1 first relative who had verified CeD. Comparison of data from 10 children who developed CeD themselves and 10 best-matched controls who did not develop disease revealed that the children who remained healthy demonstrated increased bacterial diversity over time and had a relative abundance of Firmicutes families and Bifidobacterium longum, while children who developed CeD demonstrated an increased abundance of Bifidobacterium breve and Enterococcus spp.
Another group of scientists that used 16S rRNA sequencing to investigate the microbial profile changes in children who received treatment for CeD compared to those with untreated CeD found no significant changes in alpha diversity of the microbiome at CeD diagnosis. However, in CeD individuals, they did identify a significant reduction in the abundance of Alistipes, a gram-negative rod that has protective effects against intestinal inflammation (colitis), compared to healthy controls.
Other studies have found that infants newly diagnosed with CeD have fewer Bacteroides–Prevotella, Akkermansia and Staphylococcaceae. In adults, the dominant phylum is often Firmicutes, with a higher abundance of Bacteroidetes compared to healthy controls. It is also worth mentioning that beta diversity, which reflects the compositional differences in the gut microbiome, is significantly different between CeD patients and healthy controls.
The observation that the gut microbiome composition differs amongst healthy individuals and those with CeD has been well-documented, however, many questions remain pertaining to the role(s) that the influenced species are playing in health and disease. Larger studies are needed to help answer some of these pressing questions.
Tackling Celiac Disease Through Biotherapeutics
In the realm of CeD management, adherence to a gluten-free diet (GFD) is of prime importance. However, emerging research suggests that incorporating biotherapeutics could be beneficial. Probiotics, such as Bifidobacterium and Lactobacillus, may help restore gut microbiota composition, pre-digest gluten and reduce inflammation, while prebiotics can support improved gut health and enhance the growth of beneficial microbes.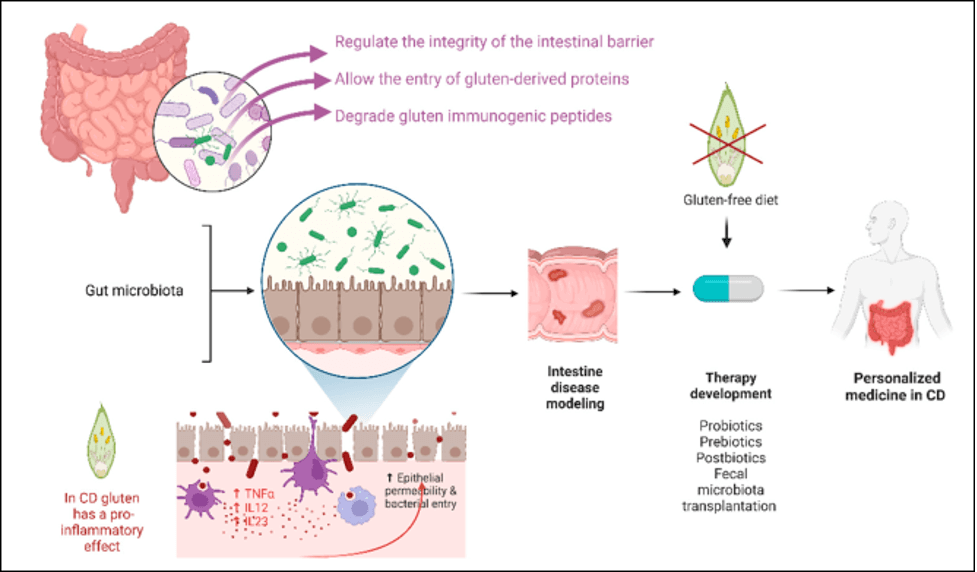

Source: Rosse, R.E. et al./Cells, March 2023
Probiotics
Probiotics are live microbes that have beneficial impacts on human health. When probiotics are administered in sufficient amounts, they alter the gut microbial profile from a disturbed (dysbiosis) to a healthy (normobiosis) state and improve host immunity. The potential impact and application of probiotics are regularly explored by scientists for alleviation of different metabolic and intestinal diseases like CeD. A group of scientists found that administration of commercial enzymes with probiotic strains of Lactobacillus and Bacillus can degrade gluten (immunogenic) to non-immunogenic and non-toxic peptides within the human gut. These findings suggest that probiotics can be used to improve the intestinal digestion of celiac patients and gluten-sensitive patients. Significant research is underway to explore the potential of probiotics in managing celiac disease (CeD). However, it’s important to note that currently, no specific probiotic has received approval from regulatory bodies such as the U.S. Food and Drug Administration (FDA) for CeD treatment.Prebiotics
Source: Iiulia Kudrina /iStock.com
Many gluten-free diets can lead to a reduction in beneficial gut bacteria. This is because most gluten-free diets have lower concentrations of fiber and nutrients that support the growth of beneficial microbiota. Adding prebiotic-rich foods or supplements helps counteract this effect and maintain a healthy gut microbial profile.
Synbiotics
The term synbiotics is used for the products that contain both probiotics and prebiotics. Examples of synbiotic food products include fermented dairy products, like yogurt, cheese, etc. Introducing synbiotics helps to improve the intestinal barrier permeability, decrease the anti-tissue transglutaminase antibody (TTGA) levels, increase the production of short chain fatty acids (SCFAs) and ultimately restore the gut microbial profile. Research on synbiotics for managing celiac disease is still in its early stages. Limited data are available regarding the effects of administering synbiotics to individuals with celiac disease.Postbiotics
Postbiotics are defined as products (molecules or compounds) obtained from live bacteria or extracted after lysis. Examples include SCFAs, lactic acid, bioactive peptides and proteins, hydrogen peroxide (H2O2), bacteriocins, organic acids and exopolysaccharides. When postbiotics are administered in adequate amounts, they help alleviate CeD symptoms. For example, postbiotics from Lactobacillus paracasei CBA L64 can reduce the gliadin-induced inflammatory response. SCFAs and Polysaccharide-A from Bacteroides fragilis have been found to reduce leaky gut and immune activation. Additionally, postbiotic enzymes like prolyl endopeptidase degrade gluten. Postbiotics are cell-free products and have higher stability and safety compared to probiotics: they have no risk of microbial infection and translocation.The Future of CeD and Microbiome Research
The intricate relationship between gut microbiota and CeD offers promising avenues for understanding and potentially managing this complex autoimmune disorder. Imbalances in the gut microbiome, characterized by a lower abundance of beneficial microbes, play a crucial role in promoting inflammation and intestinal permeability, key factors in the pathogenesis of CeD. While the primary management strategy for celiac disease remains adherence to a strict gluten-free diet, emerging research highlights the potential of biotherapeutics to complement traditional dietary approaches and offer new hope for those with CeD by targeting the underlying microbial and immunological disturbances.Future research should aim to deepen our understanding of the specific mechanisms through which gut microbiota influence the onset and progression of CeD. Longitudinal studies are needed to identify causal relationships and potential biomarkers for early diagnosis and intervention. Additionally, exploring the efficacy and safety of various biotherapeutic approaches in large-scale clinical trials will be crucial. Personalized medicine approaches, considering the unique gut microbiota composition of the individual, could also enhance the effectiveness of these treatments. Understanding the interactions between diet, the gut microbiome and immune responses will pave the way for innovative therapies, potentially transforming the management of CeD and improving patient outcomes.
Want to learn about other innovative approaches that scientists are taking to treat inflammatory disorders in the gut? Tune into this episode of Meet the Microbiologist, where we discuss how biosensors and “wearables,” like diagnostic diapers and nursing pads, could help monitor microbiome development to treat disease.
