What's New in the Fight Against Malaria?
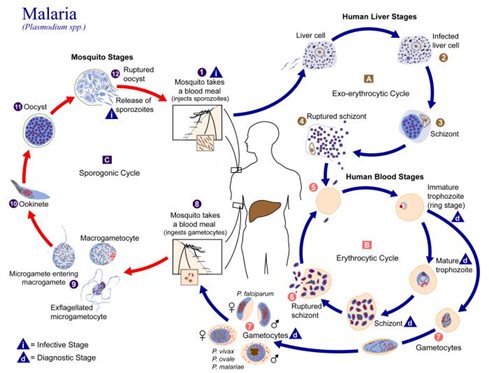
Malaria has plagued human populations for millennia. Caused by species of Plasmodium parasites, which spread to, and between, humans through the bite of Anopheles mosquitos, malaria triggers symptoms ranging from fever and nausea to organ failure and severe anemia. The disease is endemic to tropical and subtropical regions across the globe, with 95% of cases occurring in sub-Saharan Africa. Pregnant women and young children are the most vulnerable to infection—of the 627,000 deaths from malaria in 2020, most were young children in sub-Saharan Africa. If they survive, some children will suffer repeat infections.
Though there has been progress toward mitigating this deadly disease, the battle is far from over. Due in part to the COVID-19 pandemic, which disrupted disease surveillance and control efforts, the burden of malaria has risen. Yet, “even before the pandemic, progress [on controlling malaria] had sadly stalled,” Dr. Mehreen Datoo, a clinical research fellow and Ph.D. student at The Jenner Institute at the University of Oxford, said in a talk given at ASM Microbe in June 2022. The reasons for this are complicated, and include inadequate access to quality health care and funding.

Datoo emphasized that now is the time to pick up the slack. She cautioned that, without considerable action, the World Health Organization’s (WHO) 2030 targets against malaria—including reducing malaria case incidence and mortality rates globally by 90% compared to 2015, when cases and deaths reached 212 million and 429,000, respectively—will not be met.“We need to make a change.”
What Methods are Currently Used to Prevent Malaria?
Vector control tactics, like insecticide-treated nets and indoor residual spraying of insecticides, are integral to malaria control efforts; they prevent Plasmodium-harboring mosquitoes from feasting on humans. The prophylactic administration of full courses of anti-malarial drugs, known as chemoprevention, is another technique used to thwart infections. Various chemoprevention programs exist, including intermittent preventive treatment in pregnancy and seasonal malaria chemoprevention, which is given to children under 5 years of age during the malaria season.
These management strategies are not perfect. The emergence of insecticide-resistant mosquitos (78 countries reported resistance to at least 1 commonly used insecticide between 2010 and 2019), could dampen the efficacy of existing vector control methods. For example, chemoprevention may select for drug-resistant parasites, which could limit its power in the future. Taking steps toward eliminating—and, perhaps one day, eradicating—malaria will require expanding and diversifying the collection of tools within the world’s malaria-fighting toolkit.New Tools: Vaccines, Drugs and More
Vaccines
For Datoo, promising change comes in the form of vaccines. Over the past 20 years, there has been considerable progress in the development of malaria vaccines targeting different stages of the Plasmodium lifecycle. For instance, some vaccine candidates target Plasmodium merozoites, the form of the parasite that replicates in red blood cells and causes malaria symptoms.
Other vaccines, called pre-erythrocyte vaccines, target proteins on Plasmodium sporozoites residing in the liver, thus halting their eventual transformation into merozoites that enter the bloodstream. The most successful malaria vaccine to date, RTS,S/AS01, is of the pre-erythrocyte variety. It targets circumsporozite protein (CSP), the dominant surface protein on Plasmodium sporozoites that plays a key role in sporozoite invasion of liver cells.
Results from a 2009-2014 Phase III trial demonstrated that 4 doses of RTS,S/AS01 showed 36.3% reduction in malaria cases in children 5-17 months of age over a 3-4-year period. Follow-up studies examining vaccine efficacy in 500,000 children in Malawi, Ghana and Kenya revealed a similar trend, with a 30% and 21% reduction in severe malaria and hospitalizations, respectively.
“When you think about the numbers of children who die of malaria each year, these numbers make an impact,” Datoo said.
In October 2021, WHO recommended RTS,S/AS01 for broader use in children living in regions with moderate to high malaria transmission. However, Datoo noted, “at the moment, there is the worry that supply may be an issue.” The maximum production capacity of 15 million RTS,S/AS01 doses per year falls far below the expected demand of over 80 million doses annually. Development of additional vaccines will be important to fill in the gaps. One promising example is R21/Matrix M, a pre-erythrocytic vaccine also targeting CSP that Datoo shepherded through the clinical trial pipeline. In a Phase III trial including 4,800 children in 4 African countries, the vaccine exhibited an average efficacy of 68-75% for preventing clinical malaria up to 12 months after vaccination. Based on the promising data, in October 2023, WHO recommended the R21/Matrix M for prevention of malaria in children; the vaccine is expected to become available to countries in mid-2024.
Drugs
Though vaccines have captured the most attention as new, viable tools for malaria prevention, there have also been developments in the field of preventive drugs. For example, a single-dose, dispersible form of the anti-malarial drug, tafenoquine, was approved by the Australian Therapeutic Goods Administration for preventing malaria caused by P. vivax (responsible for between 4 and 5 million infections each year) in children aged 2 years and up. The formulation, which can be dissolved in water, is easier for children to take than the 7-14 day course of pills approved for adults. Researchers have also identified a nucleoside sulfamate that causes enzymes within Plasmodium parasites to self-destruct. The compound showed potent parasite-killing powers in animal models and may be a steppingstone toward development of new anti-malaria drugs for humans.
Vector Control
Progress has also been made toward improving the efficacy of existing vector control strategies, such as modifying mosquito nets to combat insecticide resistance, as well as developing new tactics, like genetically modifying mosquitos to induce sterility and an inability to bite humans. Moreover, it was demonstrated that Anopholes mosquitoes (the species that spread malaria) can be naturally infected with bacteria belonging to the Wolbachia genus. In Aedes mosquitoes, the vector for yellow fever, Dengue fever and Zika fever, Wolbachia inhibit the mosquitoes’ ability to transmit the viruses that causes these deadly infections. In fact, Wolbachia-infected Aedes mosquitoes are being deployed across the world as a disease control tactic. Could a similar approach be used for malaria? Though it was long-believed Anopholes mosquitoes did not harbor Wolbachia bacteria, the finding that they can may open doors for new preventive tactics. More research is required to determine if, and how well, Wolbachia can prevent malaria transmission.
Moving Forward
Is a future without malaria possible? Strategies to combat the disease are as complex as the lifecycle of the parasite that causes it. One thing is clear: there is no time to waste.
“My talk has been half an hour,” Datoo said. “And, in that time, 50 children have died from malaria.”
Ultimately, a multi-step, multi-faceted approach using old and new methodologies, coupled with improved surveillance of malaria cases and changes in transmission, will be required to reduce the disease burden across the globe.
Research in this article was presented at ASM Microbe, the annual meeting of the American Society for Microbiology, held June 9-13, 2022, in Washington, D.C.
Peering into history can help put recent malaria cases in context. Lessons learned can be applied to other areas battling endemic malaria and surveillance and prevention efforts around the world.
