What You Should Know About Avian Influenza A (H5N1)
Few single pathogens have influenced human history as profoundly as the influenza viruses. They circulate with seasonal predictability and occasional devastation, so it is easy to forget that most influenza A viruses are not human pathogens. Their natural reservoir is wild birds. The species promiscuity of the emerging avian influenza A virus (H5N1) clade 2.3.4.4b offers new insights into an ancient pathogen.
Where Did Avian Influenza A (H5N1) Clade 2.3.4.4b Come From?
Identified in Wild Birds in 2020
Influenza A (H5N1) clade 2.3.4.4b has been circulating at least since 2020 in wild birds in Europe, Asia and Africa. Next-generation sequencing (NGS)-based evidence placed its arrival in wild birds in North America in late 2021, with detection in U.S. commercial poultry flocks by February 2022. It is not unusual for avian influenza viruses (AIVs) to migrate with wild birds. Their diversity and global movement are monitored by the WHO’s Global Influenza Surveillance and Response System.
A Surprising Spillover With High Mortality in Wildlife

Appearance in Dairy Cattle
In February 2024, the U.S. Department of Agriculture (USDA's) national network of veterinarians began discussing an unusual illness affecting dairy cattle in Texas. Lactating adult cows seemed to be most affected, becoming suddenly ill with nonspecific symptoms. Sick cows ate less, and their milk production dropped dramatically, but they typically recovered within a couple of weeks. By March, farms from 2 other states reported similar illnesses in their herds. Molecular testing by RT-PCR detected influenza A (H5N1) in milk, later confirmed to belong to clade 2.3.4.4b. This event is the first time an influenza virus was known to spillover into cows.
How Did H5N1 Spillover Into Cows?
Pathogen, Host and Environmental Factors Must Align
A spillover requires a pathogen that can infect a new host, a host that is susceptible to passing on the infection and the circumstances that bring them together. Collaborative investigations between local veterinarians, public health professionals and federal officials have begun to paint a picture of how these events might have played out in the case of influenza A (H5N1).
The airspace over Texas is a nexus for 2 major flyways for migratory birds in which clade 2.3.4.4b was circulating. Typical dairy operations in the U.S. provide opportunities for inter-species interactions; many barns have open windows, and cows’ feed is attractive to birds. Additionally, free-range dairy cows share space with commercial poultry that may have been exposed to an avian virus and with small mammals, like barn cats or skunks, that prey on wild birds. AIVs replicate in the guts of infected birds and are shed in their droppings, which cows could ingest through grazing or eating contaminated feed.
Preliminary testing has found evidence of the virus in the stomachs and nasal swabs from infected cows. Once the virus entered the herd, cows were not primed to slow its spread. They do not have any natural immunity to AIV, and there is no bovine vaccine. Furthermore, cows spend most of their time grouped together and sharing milking equipment, food and water—a situation that lends itself to disease transmission.
A Single Transmission Event Into Cattle May Have Sparked the Current Outbreak

Phylogenetic analysis showed that the viruses from cows were similar to viruses in local wild bird populations, but most similar to each other. This is a pattern that would be expected from a single zoonotic spillover followed by onward transmission within the herd, instead of multiple introductions. Evolutionary modeling estimated that the original introduction from birds to cows could have happened as early as Dec. 2023 with the virus quietly circulating for months. The same analysis found that the genetic distance in viral sequences from different states mirrors known inter-state movements of cows.
By early May 2024, the CDC had reported that 2 dairy workers from Texas and Michigan, respectively, tested positive for the same virus. Both cases experienced only eye irritation and were discovered through active monitoring for unusual symptoms. AIV can cause a range of illness in people, from a mild “cold” to severe or even fatal pneumonia requiring hospitalization. Both patients rapidly received treatment with the antiviral oseltamivir and recovered uneventfully. These were the first cases in which a spillover of this virus into humans from a mammal was implicated.
Preliminary NGS analysis of the viruses from the first 2 human cases showed more similarity to virus from infected cows than from wild birds. Symptoms in the eyes, rather than respiratory symptoms, may reflect the exposure route of the dairy workers to the virus, either through touching the eyes after handling infectious milk or other materials in the barn, or through the milking process. A third case, also from a farm worker in Michigan, is now the first to report both eye discomfort and typical respiratory symptoms after working closely with sick cows.
What Virological Factors Influence the Risk of Avian Influenza Virus Transmission to Mammals?
Our understanding of the factors necessary for AIVs to replicate in mammals is incomplete. Several genomic hallmarks of host adaptation are routinely monitored globally. These data are integrated with ecological, public health and population level information to evaluate the pandemic risk of new viruses, e.g. WHO’s Tool for Influenza Pandemic Risk Assessment (TIPRA) and CDC’s Influenza Risk Assessment Tool (IRAT).
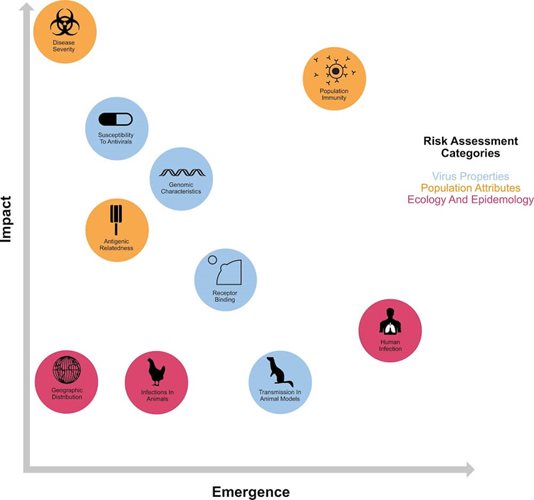
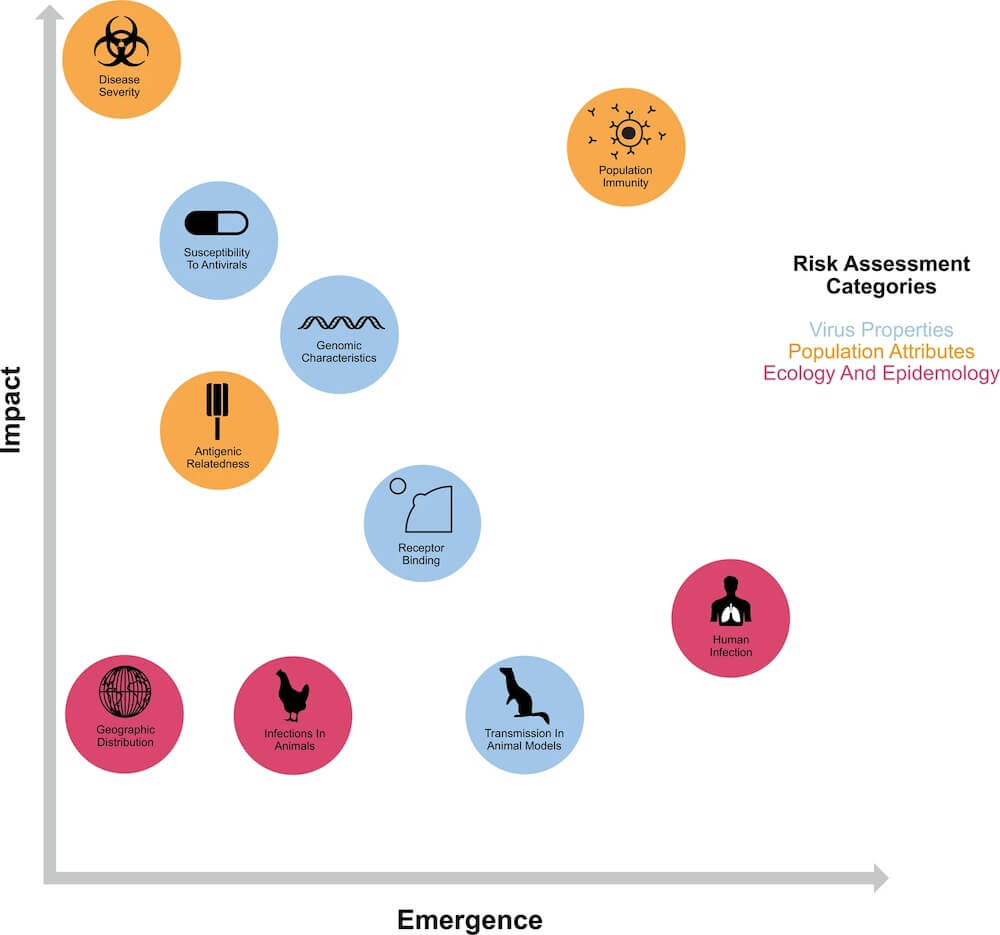
The viral requirements for spillover and effective onward spread between mammals has been the subject of critical, yet contentious research and requires careful study in animal models of respiratory transmission. Specific mutations on several gene segments (some of which are described below) influence host adaptation, and it is likely that multiple substitutions are required for spillover to occur.
Coinfection and Reassortment
Furthermore, it is common for multiple influenza viruses to co-circulate, and reassortment, the shuffling of gene segments between influenza viruses during a coinfection of the same host, can accelerate the adaptation process.
For this reason, classification of influenza viruses is both scientifically important and practical. Laboratory characterization was historically based on serological studies; viruses were grouped based on distinct patterns of reactivity with red blood cells that we now know are attributable to the 2 major viral structural proteins, hemagglutinin (HA) and neuraminidase (NA)—hence the H5N1 subtype. However, subtyping provides limited information about a virus’s ability to cause disease. AIV’s can also be classified as either “high pathogenicity” or “low pathogenicity" viruses (HPAI or LPAI) based on the extent to which they cause illness in domestic poultry.
The virus that spilled into cows in Texas was likely the product of a reassortment event in birds between a LPAI virus and a HPAI virus. The resulting virus, dubbed genotype B3.13, inherited an influenza virus nucleoprotein (NP) gene previously linked to increased transmission in commercial swine.
Changes in Receptor-Binding Domain
A key determinant of host range is a virus’ ability to bind to target cells. The HA protein of AIVs preferentially binds to glycoproteins with terminal sialic acids linked by an α2,3 linkage to the rest of the oligosaccharide chain, whereas the HA protein of viruses routinely circulating in humans preferentially binds a terminal α2,6 linkage. This preference mirrors the difference in relative abundance of each type of linkage between the avian digestive tract and the human upper respiratory tract. Both genomic and phenotypic studies from sequencing the HA gene and laboratory binding studies of the viral HA protein, respectively, of a virus isolate from the Texas outbreak showed no evidence of this virus developing a preference for the “human-like” receptors. However, sequences of viral isolates from infected dairy cattle and cats sporadically revealed some mutations known to influence binding affinity to the human-type receptor, underscoring the importance of continued monitoring.
Mutations to Viral Polymerase
Host adaptation also depends on viral polymerase activity in mammalian cells. Specifically, mutations in position 66 of the polymerase subunit PB1 and at 591, 627 and 701 of the subunit PB2 protein are associated with high pathogenicity in birds and required for efficient replication in non-avian hosts. Sequences from infected elephant seals in Argentina in 2023 carried some of these mutations.
These polymerase gene mutations were absent in the sequences from sick dairy cows and cats in Texas but, concerningly, the E627K substitution was observed in the sequence of the Texas human case. Its origin is unclear. It is possible that it emerged during replication in the patient, particularly since it has not been observed from available sequences from wild birds or from commercial poultry in the region.
Other Key Genomic Changes
The viral NA protein is important for the efficient release of new viruses from an infected cell and is, therefore, a target of licensed antivirals. Analysis of available N1 sequences has not detected a mutation associated with antiviral resistance at position 275 from dairy cows, wild birds or human cases. Similarly, mutations in other gene segments associated with antiviral resistance have not been detected.
What's Next?

Early evidence from federal authorities suggests that standard industrial pasteurization processes are adequate to inactivate any virus present in retail milk and milk products; they continue to advise against the consumption of raw milk and products prepared with raw milk, as it may contain infectious virus. Officials have advised that cooking beef to safe internal temperatures would also inactivate any virus in the unlikely event that it is present.
What is the primary mechanism of transmission between cows, and how can it be stopped? Is a genome reassortment event required for spillover of avian origin viruses to mammals, and how does this virus’ unusual propensity to transmit to diverse species impact risk for human health? Can we leverage new data from wastewater testing for influenza A virus to inform public health, agricultural and wildlife conservation actions?
Foundational laboratory test methods, like viral culture, serology and animal studies, will be critical to address these questions, as will continued investment in our public health infrastructure. Although recent data suggest that the Texas outbreak-associated viruses are not able to spread efficiently by respiratory transmission in mammals, influenza viruses have a seemingly limitless capacity to surprise us. NGS provided clues to guide our growing understanding of this new threat, and genomic surveillance will be necessary for our continued preparedness.
This is an emerging situation. Data summarized in this article are up to date as of June 25, 2024 but are subject to change.
Interested in learning more about influenza viruses and how to protect yourself from disease? Check out our Flu resource page, where you can browse all of our latest content on this subject.
