Where Did Zika Go (And Will It Come Back)?
In 1947, researchers isolated a new virus from a rhesus monkey in a tropical forest near Entebbe, Uganda. The virus, of the Flavivirus genus, was named Zika after the forest in which it was discovered. The Zika virus (ZIKV) is closely related to other mosquito-borne flaviviruses, including the well-known West Nile, dengue and Japanese encephalitis viruses.
Shortly after its discovery, ZIKV was isolated from Zika forest Aedes africanus mosquitoes. Further research determined that Aedes aegypti as well as Aedes africanus was a vector for ZIKV, passing the virus between monkeys and mice and suggesting that the mosquito could also transmit the virus to humans.
For decades, the virus remained off the global radar, but was believed to be pervasive in parts of Africa—specifically tropical regions in the middle of the continent (south of the Sahara Desert and north of Namibia, Zambia, and Mozambique). But in 2007 the Yap Islands of Micronesia, a small group of islands in the western Pacific Ocean, experienced the first major outbreak of ZIKV outside of Africa. According to a NEJM study, about 73% of the residents of the Yap Islands were infected with ZIKV.
Prior to 2007 reports of ZIKV infection described the virus causing nonspecific symptoms reminiscent of the common cold or dengue fever. This allowed the virus to hide in plain sight, causing no fatal infections or any symptoms that would distinguish it from another viral infection. There exist large numbers of viruses that infect humans but don’t cause disease, such as human hepegivirus 1, a seemingly nonpathogenic relative of Hepatitis C Virus. Thus, Zika was deemed generally benign, until questions began to arise during the Yap Islands outbreak of 2007. This specific outbreak was interesting because while the congenital symptoms of ZIKV infection were not yet observed, reports from physicians on the islands indicated that the virus was similar to dengue, but was not behaving like other clinically determined dengue infections. For the first time, ZIKV had developed novel symptoms that separated it from other general viral infections.
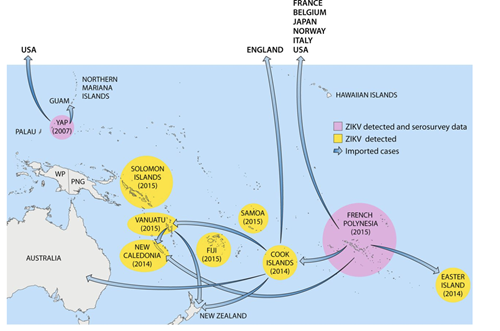
In 2013, the virus leapt to Cambodia, Thailand and then French Polynesia, where it produced several large outbreaks (Figure 1). It was in French Polynesia where health care workers first observed the unique congenital and neurological symptoms of ZIKV that drew media attention. ZIKV then moved to northeastern Brazil early in 2014, and spread through the Americas, peaking in 2016.
Zika, the "Benign" Virus, Turns Bad
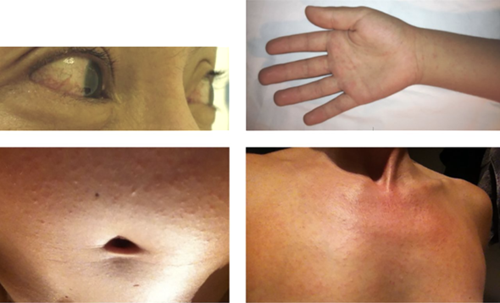
In 2014-2015, patients had been streaming into Brazilian hospitals with rashes, fevers and conjunctivitis, seemingly with some sort of mystery infection (Figure 2). Over 6,800 blood samples were tested for parvovirus, dengue and chikungunya, among others, and each was systematically ruled out. Finally, ZIKV was confirmed to be the agent responsible for the multitude of infections. Hearing this news, the Brazilian health minister at the time, Dr. Arthur Chioro, stated to reporters, “Zika virus doesn’t worry us, it’s a benign disease.” But soon after his public statement, concerning evidence to the contrary emerged.
Babies were being born with tiny heads in alarming numbers. None of the affected infants tested positive for ZIKV, but their mothers had experienced a mysterious rash, consistent with ZIKV infection, early in their pregnancies. Soon after, ZIKV was found in the amniotic fluid of one pregnant monther, as well as in the blood of two stillborn and malformed fetuses. It soon became apparent that ZIKV infection could be transmitted between mother and fetus, and was linked with newborn microcephaly (babies with unusually small heads). On February 2, 2016, ZIKV was deemed a public health emergency of international concern(PHEIC) by the World Health Organization. Its rapid expansion from Brazil throughout the Americas caused great concern as ZIKV made its way across the U.S. border and into Texas, Florida and Hawaii.
ZIKV’s quick spread was thought to be due to the Aedes mosquitoes common to South America and Central America countries as well as some southern states of the U.S. As a result, the CDC released a domestic travel advisory warning pregnant and soon-to-be pregnant women to avoid travel to Central and South America, the Caribbean and Miami-Dade county in Florida.
In the meantime, scientists and policy makers were struggling to understand why ZIKV moved into the Americas so rapidly. Dr. Michael Diamond, a microbiologist and pathologist at the Washington University School of Medicine, believes that ZIKV moved into a new niche where humans were completely immunologically naive, allowing the virus to spread like wildfire among people whose immune systems had never encountered the virus before. It is probable that as the virus moved from monkey to human, it evolved to better fill its new niche, and that the virus became more virulent as it adapted to the human immune system. This in part explains why the ZIKV infection symptoms appeared to change as the virus spread from Africa to Asia to South America.
Zika Falls Off the Radar - But Why?
In 2017, reports of ZIKV outbreaks reduced drastically and infection numbers today are markedly down from the 2016 peak. Thus far in 2018, the CDC has reported only 34 cases of ZIKV in the U.S., all from travelers returning to the U.S. from Zika affected areas. Compare that to the 5,168 symptomatic ZIKV disease cases reported in 2016. In the U.S. territories of Puerto Rico, American Samoa, and the U.S. Virgin Islands, there have only been 74 symptomatic ZIKV cases in 2018, compared to 36,512 cases reported in 2016. Even in South America, ZIKV infections have significantly dropped—from over 30,000 suspected and confirmed cases in early 2016 to an average of 293 in the last half of 2017.
Experts like Diamond say the swift drop in ZIKV cases is likely due to the development of herd immunity. When ZIKV first spread from central Africa, humans were immunologically naive and didn’t have natural defenses against the viral infection. Now, two years after the peak, enough people have become immune to the virus, somewhat like the immunity gained after a bout of chickenpox, to halt the ZIKV transmission chain.
"If a large enough proportion of the herd—be it cows or mice or people—are resistant to a disease, it's very difficult for the disease to spread," Dr. Uriel Kitron, an expert in viruses transmitted by mosquitoes at Emory University, told the Chicago Tribune last year, when ZIKV numbers were first beginning to drop.
Many people who become infected with ZIKV don’t have symptoms, or have symptoms closely resembling those of the common cold. Thus, areas where the disease was endemic may have large populations of people who were unknowingly infected and are now immune. This prevents the virus from moving from person to person, even in an area teeming with mosquitoes. ZIKV transmission requires mosquitoes to bite people with active disease, so the insect can carry the virus to a new host. If few individuals produce an active infection, the probability of a mosquito biting someone with active disease and transmitting it to one of the few susceptible individuals in the population drops precipitously.
What is the Future of Zika Virus?
"But don’t get too comfortable just yet," says Dr. Scott Weaver, Director of the Institute for Human Infections and Immunity at University of Texas Medical Branch in Galveston, Texas. He says there simply isn’t enough surveillance of the virus in South America due to lack of resources, so the absence of large outbreaks doesn’t necessarily mean a complete absence of ZIKV transmission. Furthermore, because the virus is constantly mutating, herd immunity only lasts for a limited time.
Even if ZIKV were to suddenly cease to exist, concerns for those who had been infected or born to infected mothers would remain. Only 6% of pregnancies where the mother was exposed to ZIKV resulted in obvious birth defects. What that means for the other 94% of ZIKV-exposed pregnancies remains unclear. ZIKV targets neurons, and researchers are trying to uncover how the virus affects the development of babies born from ZIKV-infected mothers. Only time will tell if these children will develop normally.
ZIKV can also affect adults long after the infection subsides. A rare autoimmune nervous system condition called Guillain-Barre syndrome can be triggered if ZIKV infects neurons. The affected person’s immune system wrongly attacks its own neurons, causing a wide variety of symptoms ranging from mild weakness to complete body paralysis. In April of this year, the Brazilian Journal of Infectious Diseases published ameta-analysis on the incidence of Guillain-Barre syndrome in association with ZIKV infection. Researchers concluded that there have been about 1,500 cases of ZIKV-associated Guillain-Barre syndrome—roughly translating to a rate of less than 1% of all ZIKV infections, but there may be many more unreported cases of people who didn’t know they were infected with ZIKV.
Zika Research Leads Scientists to Mosquitos, Microbes and Vaccines
In the past 2 years, the scientific community has answered important questions regarding ZIKV virulence, viral evolution and immune system evasion. In June, researchers produced the highest-resolution image of the ZIKV virus (or any flavivirus) yet, leading them to better understand the three-dimensional structure and biophysics of the virus. Scientists are beginning to unravel how ZIKV causes congenital disease, but many unanswered questions remain, including what are the dynamics of ZIKV transmission, how ZIKV persists and how it reacts with related viruses.
In 2016, the international ZIKV epidemic and subsequent panic led the United States to quickly divert $1.1 billion into ZIKV research over two years. Now it is 2018, and many of the R21 grants awarded in 2016 to study ZIKV are expiring. Furthermore, reduction in ZIKV outbreaks means that many grants won’t be renewed this year. So, where does this leave ZIKV research, particularly vaccine development?
There’s some good news. The National Institute of Allergy and Infectious Disease has developed several ZIKV vaccine candidates, one of which is currently in phase 2 clinical trials in Texas, Puerto Rico, South and Central America.
Another approach to fighting current and possible future ZIKV infections is targeting the vector, Aedesmosquitoes. Using insecticides, which seems like an obvious solution, dramatically impacts other organisms occupying ecological niches in which Aedes mosquitoes reside. Rather, scientists and ecologists of the World Mosquito Program favor alternate solutions, such as using Wolbachia bacteria. Certain Wolbachia strains , when introduced into Aedes mosquitoes, block the transmission of ZIKV, dengue and Chikungunya virus. The bacteria are transmitted to the next generation of mosquitoes within the eggs, so the program is self-sustaining. A successful trial has eliminated dengue cases in Townsville, Australia, and similar strategies may work to eliminate the spread of ZIKV by mosquitoes.
Will Zika Virus Come Back?
Unfortunately, based on the behavior of other flaviviruses, we can probably expect ZIKV to resurface. In the case of West Nile Virus, outbreaks stopped appearing for a couple of years, but then returned; with dengue, infections increase every summer, tracking the increase of the mosquito population. Researchers are not yet sure what the trajectory of ZIKV will be but caution people to remain vigilant, particularly pregnant women and those traveling into endemic areas.
Further Reading and Media Links:
American Society for Microbiology - Zika Virus Press Conference 2016
Wolbachia wStri Blocks Zika Virus Growth at Two Independent Stages of Viral Replication
Musso D. and Gubler D.J. Zika Virus. Clinical Microbiology Reviews. 2015
