Why Do Some Vaccines Work Better Than Others?
If someone is vaccinated against the measles virus, they likely won’t get measles.
If someone is vaccinated against SARS-CoV-2, there’s still a good chance they’ll get COVID-19—maybe multiple times.
And how much it's spreading.
And the type of vaccine.
And the immune response stimulated by the vaccine.
In short, it’s complicated.
What is clear is that it’s less about whether vaccines are good or bad (note: they are, in principle, very good), and more about defining what we should expect of them.
I Got Vaccinated—So Why Am I Sick?
Vaccines help the body recognize pathogens as foreign entities and quickly mount an attack against them. Often, people think this means vaccines are designed to prevent infection, or facilitate elimination of a pathogen before it colonizes its host (known as “sterilizing immunity”). This has led to the misconception—fueled in part by unclear messaging on the part of scientists and media—that if someone gets sick with a pathogen they were vaccinated against, the vaccine failed. While sterilizing immunity would be an ideal outcome, it is not currently attainable. Instead, vaccines are largely geared toward preventing disease (i.e., the outcome of infection), which can also help minimize the spread of the virus.
With that in mind, some vaccines do an excellent job of preventing disease, though no vaccine can so 100% of the time. Many vaccines simply prevent severe disease—they may not, and were never meant to, stop sickness altogether. They can, however, keep a disease from being much worse than it might be without vaccination. A vaccine can also curb hospitalizations, which is not only good for the vaccinated person, but also eases burdens on health care systems. Moreover, vaccines are safer than immunity gained through natural infections, which can lead to chronic conditions (e.g., long COVID, long flu, etc.)
The type of protection a vaccine affords, and thus what to expect of it, is determined by scientists, based on clinical trials, and by analyzing how the vaccine performs “in the real world.” It is rooted in a slew of interacting factors related to the virus, the type of vaccine, when and how often it is administered and more.
To understand how these factors influence vaccine responses, let’s look at a few examples.
COVID-19 Vaccines: Prevent Severe Disease
The Virus
SARS-CoV-2, the virus that causes COVID-19, is a single-stranded RNA virus. These viruses are notoriously sloppy replicators. They are prone to developing random mutations as they replicate in a population, meaning SARS-CoV-2 mutations happen pretty regularly, and their severity depends on where in the genome they occur. Some of those mutations become “fixed” if they help the pathogen evade immunity and transmit between hosts. For SARS-CoV-2, mutations in the spike protein, which the virus uses to infect host cells and COVID-19 vaccines are designed to target, can lead to variants that circumvent vaccine-induced immunity.
The Vaccine
Most COVID-19 vaccines are mRNA vaccines. These vaccines are given as an intramuscular injection and contain the genetic code (mRNA) to the SARS-CoV-2 spike protein. Host cells use this code to produce copies of the viral protein, which immune cells memorize and will respond to if they are exposed to the viral protein in the future. Ultimately, COVID-19 mRNA vaccines trigger a robust antibody response that wanes significantly over several months.
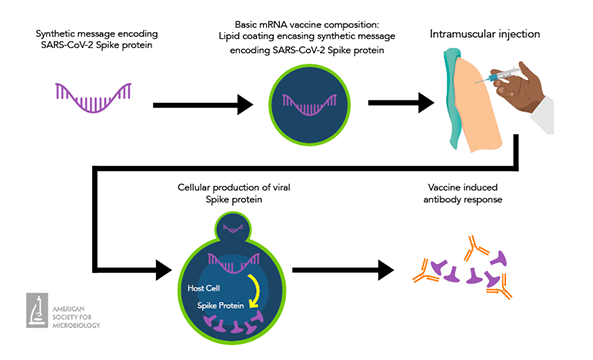
Administration
New vaccine formulations that target dominant SARS-CoV-2 variants are regularly released, with the vaccination schedule reflecting this. According to the U.S. Centers for Disease Control and Prevention (CDC), everyone should receive at least 1 dose of the most updated COVID-19 vaccine (based on the XBB.1.5 variant), with variations based on previous vaccination status, age and more.
Expectation
When the COVID-19 vaccines first came out, clinical trials suggested they were incredibly effective at preventing illness. However, those expectations were revised as the vaccines were widely deployed (the first time this was done with mRNA vaccines), and the virus continued to mutate. Further research has shown that vaccines are most effective in the first 6 months following vaccination, with a 2023 meta-analysis suggesting that 6 months after primary vaccination COVID-19 mRNA vaccines are less than 20% effective. Today, the vaccines’ primary purpose—and something they do better long-term—is to protect against severe COVID-19 that can lead to hospitalization and death.
Human Papillomavirus (HPV) Vaccines: Protect Against Cancer
The Virus
Human papillomavirus (HPV) is a small DNA virus. Most HPV types are transmitted sexually, and some cause cancer, namely cervical cancer. Notably, vaccine-induced immunity against HPV is relatively stable. This is, in part, because host DNA polymerase, which the virus uses for replication, does not tolerate errors the way RNA polymerase (used by ssRNA viruses) does. As a result, random mutations rarely become fixed.
The Vaccine
HPV vaccines are composed of virus-like particles (VLPs). These VLPs consist of capsid proteins from 2, 4 or 9 different HPV types that cause cancer, depending on the vaccine. The proteins self-assemble into particles that mimic the size and shape of a virus but are not infectious or replicative. This structural similarity to “real” virus means that VLPs induce a durable and long-lasting immune response, with high levels of neutralizing antibodies against the vaccine HPV types.
Administration
HPV vaccination begins in early adolescence; the goal is to protect people against the virus prior to exposure. The vaccine is administered via intramuscular injection. People receive 2-3 doses, depending on the age they received their first dose. This is to ensure robust immune response, as younger age (9-12 years old) is associated with a higher antibody response to the vaccines. Booster doses are not needed.
Expectation
The HPV vaccines pack a punch—according to the CDC, they can prevent more than 90% of cancers caused by HPV. The vaccines also minimize infections by the 2 most prevalent, high-risk HPV types (HPV-16 and HPV-18). This could be because the beginning of the HPV life cycle is slow to start, requiring few neutralizing antibodies to combat the virus. While people can expect high protection against HPV vaccine types, infections are still possible. Taking other preventive steps (e.g., regular cervical cancer screenings) is important.
Flu Vaccines: Take Disease from "Wild to Mild"
The Virus
The Vaccine
There are different types of flu vaccines, all of which are similarly effective. Some vaccines contain influenza viruses that have been chemically disrupted (inactivated), so they cannot replicate. Others, known as subunit vaccines, contain only purified virus antigen (proteins that elicit an immune response). Because the latter types only contain part of the virus, they may not trigger as strong of an immune response as a whole virus. There are vaccine formulations that contain higher amounts of antigen or an adjuvant (a compound that enhances an immune response) to address this.
Administration
Given the shiftiness of the flu virus, and waning immunity, it is recommended that everyone 6 months of age and older get 1 flu vaccine every year before flu season begins (i.e., by the end of October), with some exceptions.
Expectation
When developing flu vaccines, scientists survey the influenza variants circulating in the human population and select the 4 they think will be dominant come flu season. Sometimes their educated guesses are better than others, meaning someone may get flu 1 year but not the next. If there is a good match between the flu vaccine and spreading influenza viruses, vaccines are about 40-60% effective at preventing flu. However, should a vaccinated person get the flu, the illness will likely be less severe than if they weren’t vaccinated—i.e., the disease goes from “wild to mild.”
Measles (MMR) Vaccine: Nearly 100% Effective at Staving Off Disease
The Virus
Measles virus is a highly contagious respiratory virus currently spreading across the U.S., the U.K. and other countries around the world. Compared to some other viruses, measles has a notable attribute: it is unlikely to evade vaccine-induced immunity. It’s not that measles doesn’t mutate—like other ssRNA viruses, it does. Rather, the surface proteins needed for measles to infect cells (and used by immune cells to detect the pathogen) do not tolerate mutations—that is, mutations render the proteins ineffective, which is detrimental to the virus’ ability to infect host cells. Infectious virus therefore remains recognizable to the host immune system.
The Vaccine
Administration
The MMR vaccine is generally administered (once again by intramuscular injection) via a 2-dose regimen during childhood, starting at 12-15 months of age. Adults who do not have evidence of immunity can also get vaccinated. Unlike other types of vaccines, booster doses are not usually necessary—immunity is considered to be lifelong.
Expectation
The stability of the measles virus, coupled with the vaccine type, make for a stellar combination. Two doses of the MMR vaccine are 97% effective at preventing measles for life (though breakthrough infections can happen in very rare cases). When measles outbreaks occur, cases are largely concentrated in unvaccinated populations.
Ultimately, what it means to be “protected” by a vaccine is shaped by how much a virus mutates (and where in the genome those mutations occur), how closely a vaccine mimics natural infection (and thus triggers robust, long-lasting immunity), vaccine formulation and how frequently it is administered.
The Significance of Viral Spread
But there is 1 thing that holds true across all vaccines: how much a virus is spreading also impacts how “well” the vaccine works. This is somewhat intuitive. If a virus is running rampant in a population, more people are likely to get sick. Vaccines may help, but the magnitude of circulation, and how immune-evasive the virus may be, simply creates more opportunities for infection.
The prevalence of a virus can change over time. Influenza, for example, has seasonal peaks and valleys. This doesn’t mean the influenza virus isn’t around outside of flu season. What it does mean is that even with vaccines, the virus is spreading so much more during the colder months that more people will inevitably fall ill. The same idea applies for diseases with high vaccine-induced protection, like measles. In the setting of an outbreak, even if only a small number of vaccinated individuals become infected with measles (about 3 in 100 people who received 2 doses of the MMR vaccine develop measles if exposed to the virus), this can account for a large number of cases.
Depending on the virus, the more hosts a virus infects, the more opportunities for immune-evasive variants to arise and spread. This also helps dictate how well existing vaccines can train the immune system to deal with the virus.
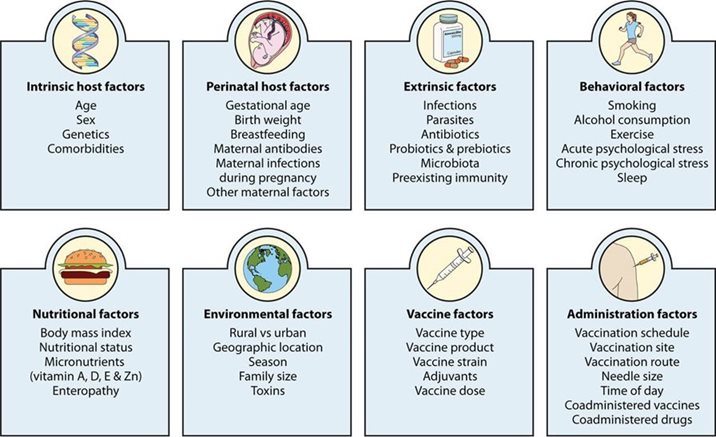
The Host Matters Too
Scientists study how effective a vaccine is on the scale of populations and communities, but on the scale of individuals, results can vary. Indeed, there are numerous factors that shape how a person responds to vaccines, including age, genetics, the gut microbiota, stress, exercise frequency, sleep patterns and more. Having certain comorbidities (e.g., those affecting the kidney, heart and lungs) can also make a difference. It’s worth noting that immunity naturally decreases over time, which is among the reasons why older populations are more vulnerable to disease. Coupled with other factors (virus type and spread, vaccine formulation and more), this can happen more quickly for some vaccines than others.
How to Think About Vaccines
It would be great if every vaccine prevented a person from getting sick. The reality, and something most people already know first-hand, is that most vaccines don’t work that way. But that doesn’t mean they don’t work at all. The bottom line: all vaccines are protective. The key is understanding—and, on the part of scientists, communicating—what protection means in the context of each vaccine, and shaping expectations accordingly.
